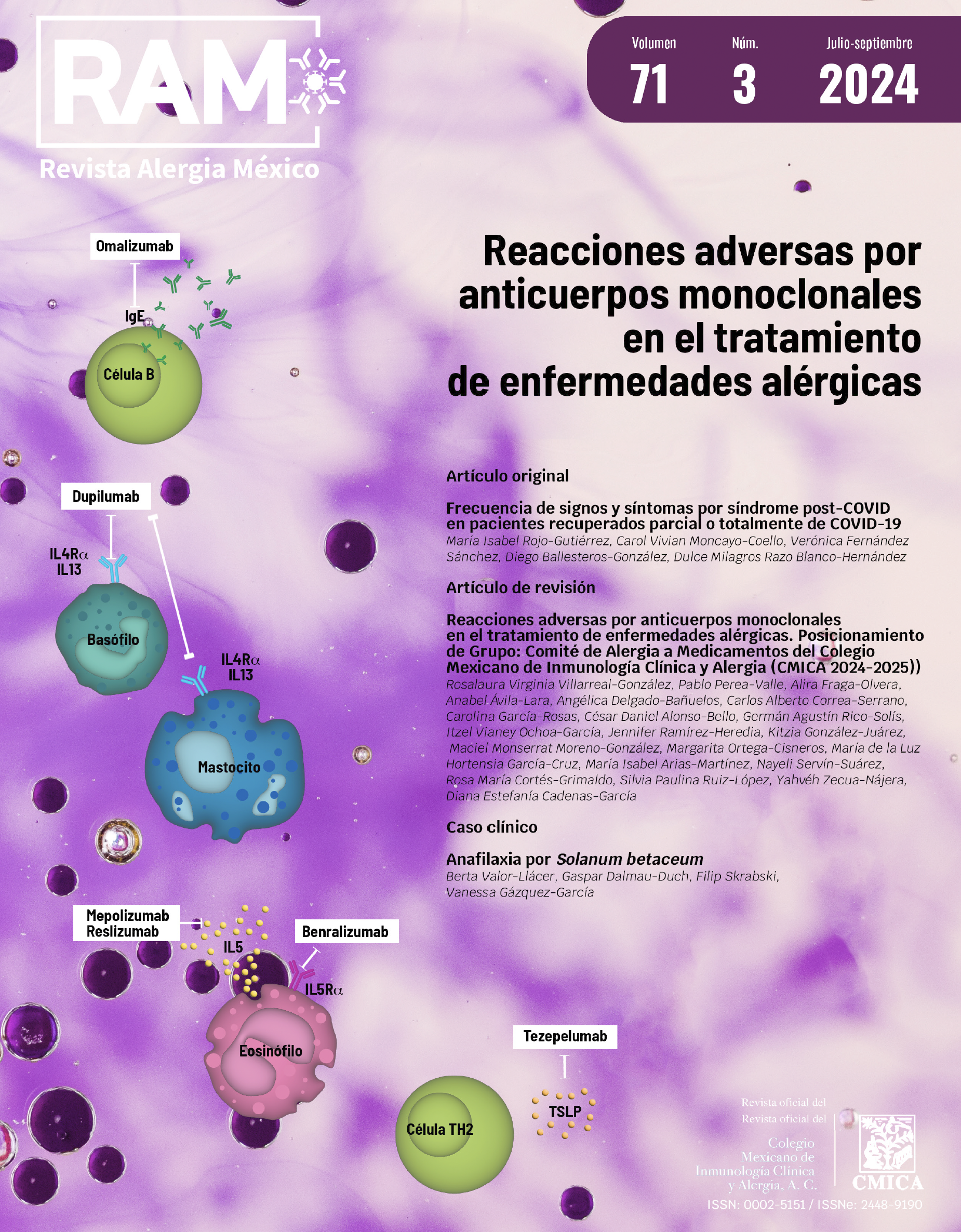
Abstract
Background: Monoclonal antibodies are a therapeutic option for allergy, autoimmune, oncological diseases, among others, and work by inhibiting interactions between effector molecules and their specific receptors. However, the increasing use of these drugs has led to an increase in adverse drug reactions (ADRs). ADRs are defined as unexpected responses to therapeutic treatments. They are divided into type A, derived from the pharmacokinetics of the treatment or as a host immune response type B.
Objectives: Review of the use of monoclonal antibodies in the treatment of allergic diseases, addressing key aspects such as pharmacokinetics, pharmacodynamics, posology, contraindications and adverse reactions.
Methods: A search of major medical databases on monoclonal antibodies for the treatment of allergic diseases was conducted. It was limited to original articles in English and Spanish, published between 2014 and 2024.
Results: Monoclonal antibodies for allergic diseases are described, including their mechanism of action, trade name, indications, posology, contraindications and adverse reactions.
Conclusion: The collection of data on biological drugs is crucial for a comprehensive and up-to-date understanding of their clinical use. Understanding adverse reactions improves diagnosis and the quality of medical care.
Keywords: Monoclonal antibodies; Adverse reactions to drugs; Hypersensitivity reactions; Pharmacokinetics; Pharmacodynamics; Posology; Contraindications; Adverse reactions.
References
WHO Expert Committee on Biological Standardization. World Health Organ Tech Rep Ser 2014; (987): 1-266.
2. Mori F, Saretta F, Bianchi A, et al. Hypersensitivity Reactions to Monoclonal Antibodies in Children. Medicina (Buenos Aires) 2020; 56 (5): 232. doi: 10.3390/medicina56050232
3. Ozdemir C. Monoclonal Antibodies in Allergy; Updated Applications and Promising Trials. Recent Pat Inflamm Allergy Drug Discov 2015; 9 (1): 54-65. doi: 10.2174/1872213X09666150223115303
4. Zazzara MB, Palmer K, Vetrano DL, Carfì A, Onder G. Adverse drug reactions in older adults: a narrative review of the literature. Eur Geriatr Med 2021; 12 (3): 463-473. doi: 10.1007/s41999-021-00481-9
5. Pichler WJ. Immune pathomechanism and classification of drug hypersensitivity. Allergy Eur J Allergy Clin Immunol 2019; 74 (8): 1457-1471. doi: 10.1111/all.13765
6. Corominas M, Gastaminza G, Lobera T. Hypersensitivity reactions to biological drugs. J Investig Allergol Clin Immunol 2014; 24 (4): 212-225.
7. Isabwe GAC, Garcia Neuer M, de las Vecillas Sanchez L, Lynch D-M, et al. Hypersensitivity reactions to therapeutic monoclonal antibodies: Phenotypes and endotypes. J Allergy Clin Immunol 2018; 142 (1): 159-170.e2. doi: 10.1016/j.jaci.2018.02.018
8. Food and Drug Administration. XOLAIR® (omalizumab) injection for subcutaneous use initial U.S. Approval 2003. XOLAIR® (omalizumab) injection for subcutaneous use initial U.S. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/103976s5225lbl.pdf
9. Purizaca-Bazán J, Ortega-Martell JA. Bloqueo de inmunoglobulina E en el asma grave. Rev Alerg México 2020; 67. doi: 10.29262/ram.v67i7.777
10. Kavati A, Zhdanava M, Ortiz B, et al. Retrospective Study on the Association of Biomarkers With Real-world Outcomes of Omalizumab-treated Patients With Allergic Asthma. Clin Ther 2019; 41 (10): 1956-1971. doi: 10.1016/j.clinthera.2019.07.021
11. Casale TB, Luskin AT, Busse W, et al. Omalizumab Effectiveness by Biomarker Status in Patients with Asthma: Evidence From PROSPERO, A Prospective Real-World Study. J Allergy Clin Immunol Pract 2019; 7 (1): 156-164.e1. doi: 10.1016/j.jaip.2018.04.043
12. Canonica GW, Rottoli P, Bucca C, et al. Improvement of patient-reported outcomes in severe allergic asthma by omalizumab treatment: the real life observational PROXIMA study. World Allergy Organ J 2018; 11: 33. doi: 10.1186/s40413-018-0214-3
13. Bhutani M, Yang WH, Hébert J, de Takacsy F, et al. The real world effect of omalizumab add on therapy for patients with moderate to severe allergic asthma: The ASTERIX Observational study. Fehrenbach H, ed. PLoS One 2017; 12 (8): e0183869. doi: 10.1371/journal.pone.0183869
14. Damask C, Chen M, Holweg CTJ, Yoo B, et al. Defining the Efficacy of Omalizumab in Nasal Polyposis: A POLYP 1 and POLYP 2 Subgroup Analysis. Am J Rhinol Allergy 2022; 36 (1): 135-141. doi: 10.1177/19458924211030486
15. Seetasith A, Holden M, Hetherington J, et al. Real-world outcomes in patients with chronic spontaneous urticaria receiving omalizumab: insights from a clinical practice survey. Curr Med Res Opin 2024: 1-12. doi: 10.1080/03007995.2024.2354534
16. Wood RA, Togias A, Sicherer SH, et al. Omalizumab for the Treatment of Multiple Food Allergies. N Engl J Med 2024; 390 (10): 889-899. doi: 10.1056/NEJMoa2312382
17. Chebani R, Lombart F, Chaby G, et al. Omalizumab in the treatment of bullous pemphigoid resistant to first-line therapy: a French national multicentre retrospective study of 100 patients. Br J Dermatol 2024; 190 (2): 258-265. doi: 10.1093/bjd/ljad369
18. Ferri N, Bellosta S, Baldessin L, Boccia D, et al. Pharmacokinetics interactions of monoclonal antibodies. Pharmacol Res 2016; 111: 592-599. doi: 10.1016/j.phrs.2016.07.015
19. C K. Omalizumab. 2023. Checar Referencia completa
20. Dantzer JA, Wood RA. Update on omalizumab in allergen immunotherapy. Curr Opin Allergy Clin Immunol 2021; 21 (6): 559-568. doi: 10.1097/ACI.0000000000000781
21. Namazy J, Cabana MD, Scheuerle AE, et al. The Xolair Pregnancy Registry (EXPECT): The safety of omalizumab use during pregnancy. J Allergy Clin Immunol 2015; 135 (2): 407-412. doi: 10.1016/j.jaci.2014.08.025
22. Namazy JA, Blais L, Andrews EB, et al. Pregnancy outcomes in the omalizumab pregnancy registry and a disease-matched comparator cohort. J Allergy Clin Immunol 2020; 145 (2): 528-536.e1. doi: 10.1016/j.jaci.2019.05.019
23. Anderson PO. Monoclonal Antibodies During Breastfeeding. Breastfeed Med 2021; 16 (8): 591-593. doi: 10.1089/bfm.2021.0110
24. Saito J, Yakuwa N, Sandaiji N, et al. Omalizumab concentrations in pregnancy and lactation: A case study. J Allergy Clin Immunol Pract 2020; 8 (10): 3603-3604. doi: 10.1016/j.jaip.2020.05.054
25. Cox L, Platts-Mills TAE, Finegold I, Schwartz LB, et al. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J Allergy Clin Immunol 2007; 120 (6): 1373-1377. doi: 10.1016/j.jaci.2007.09.032
26. Harrison RG, MacRae M, Karsh J, Santucci S, et al. Anaphylaxis and serum sickness in patients receiving omalizumab: reviewing the data in light of clinical experience. Ann Allergy, Asthma Immunol 2015; 115 (1): 77-78. doi: 10.1016/j.anai.2015.04.014
27. Bagnasco D, Canevari RF, Del Giacco S, et al. Omalizumab and cancer risk: Current evidence in allergic asthma, chronic urticaria, and chronic rhinosinusitis with nasal polyps. World Allergy Organ J 2022; 15 (12): 100721. doi: 10.1016/j.waojou.2022.100721
28. Harb H, Chatila TA. Mechanisms of Dupilumab. Clin Exp Allergy 2020; 50 (1): 5-14. doi: 10.1111/cea.13491
29. Matsunaga K, Katoh N, Fujieda S, Izuhara K, et al. Dupilumab: Basic aspects and applications to allergic diseases. Allergol Int 2020; 69 (2): 187-196. doi: 10.1016/j.alit.2020.01.002
30. Food and Drug Administration. DUPIXENT ® (dupilumab) inyection for subcutaneous use Initial. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761055s042lbl.pdf
31. Sitek AN, Li JT, Pongdee T. Risks and safety of biologics: A practical guide for allergists. World Allergy Organ J 2023; 16 (1): 100737. doi: 10.1016/j.waojou.2022.100737
32. Gade A. Dupilumab. StatPearls. 2024. https://www.ncbi.nlm.nih.gov/books/NBK585114/
33. Khamisy-Farah R, Damiani G, Kong JD, Wu J-H, et al. Safety profile of Dupilumab during pregnancy: a data mining and disproportionality analysis of over 37,000 reports from the WHO individual case safety reporting database (VigiBaseTM). Eur Rev Med Pharmacol Sci 2021; 25 (17): 5448-5451. doi: 10.26355/eurrev_202109_26652
34. Bosma AL, Gerbens LAA, Middelkamp‐Hup MA, Spuls PI. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol 2021; 46 (6): 1089-1092. doi: 10.1111/ced.14725
35. Adam DN, Gooderham MJ, Beecker JR, et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J Eur Acad Dermatology Venereol 2023; 37 (6): 1135-1148. doi: 10.1111/jdv.18922
36. Wechsler ME, Ford LB, Maspero JF, et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med 2022; 10 (1): 11-25. doi: 10.1016/S2213-2600(21)00322-2
37. Albader SS, Alharbi AA, Alenezi RF, Alsaif FM. Dupilumab side effect in a patient with atopic dermatitis: a case report study. Biol Targets Ther 2019; 13: 79-82. doi: 10.2147/BTT.S195512
38. Partida-Gaytán A, Torre-Bouscoulet L, Macías MP, Raimondi A, et al. Mepolizumab para el tratamiento de asma grave eosinofílica. Rev Alerg Méx 2021; 67. doi: 10.29262/ram.v67i7.780
39. Food and Drug Administration. NUCALA (Mepolizumab): Highlights of prescribing information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125526Orig1s021,761 122Orig1s011Corrected_lbl.pdf
40. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380 (9842): 651-659. doi: 10.1016/S0140-6736(12)60988-X
41. Moore WC, Kornmann O, Humbert M, et al. Stopping versus continuing long-term mepolizumab treatment in severe eosinophilic asthma (COMET study). Eur Respir J 2022; 59 (1): 2100396. doi: 10.1183/13993003.00396-2021
42. Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021; 9 (10): 1141-1153. doi: 10.1016/S2213-2600(21)00097-7
43. Steinfeld J, Bradford ES, Brown J, et al. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol 2019; 143 (6): 2170-2177. doi: 10.1016/j.jaci.2018.11.041
44. Roufosse F, Kahn J-E, Rothenberg ME, et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: A phase III, randomized, placebo-controlled trial. J Allergy Clin Immunol 2020; 146 (6): 1397-1405. doi: 10.1016/j.jaci.2020.08.037
45. Ullmann N, Peri F, Florio O, et al. Severe Pediatric Asthma Therapy: Mepolizumab. Front Pediatr 2022; 10. doi: 10.3389/fped.2022.920066
46. Liu AY. Infectious Implications of Interleukin-1, Interleukin-6, and T Helper Type 2 Inhibition. Infect Dis Clin North Am 2020; 34 (2): 211-234. doi: 10.1016/j.idc.2020.02.003
47. Dighriri IM, Alnughaythir AI, Albesisi AA, et al. Efficacy and Safety of Mepolizumab in the Management of Severe Eosinophilic Asthma: A Systematic Review. Cureus 2023. doi: 10.7759/cureus.49781
48. Middleton PG, Gade EJ, Aguilera C, et al. ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases. Eur Respir J 2020; 55 (2): 1901208. doi: 10.1183/13993003.01208-2019
49. Vittorakis SK, Giannakopoulou G, Samitas K, Zervas E. Successful and safe treatment of severe steroid depended eosinophilic asthma with mepolizumab in a woman during pregnancy. Respir Med Case Reports 2023; 41: 101785. doi: 10.1016/j.rmcr.2022.101785
50. Ozden G, Pınar Deniz P. May mepolizumab used in asthma correct subfertility? Ann Med. 2021; 53 (1): 456-458. doi: 10.1080/07853890.2021.1900591
51. Domingo Ribas C, Carrillo Díaz T, Blanco Aparicio M, et al. Real World Effectiveness and Safety of Mepolizumab in a Multicentric Spanish Cohort of Asthma Patients Stratified by Eosinophils: The REDES Study. Drugs 2021; 81 (15): 1763-1774. doi: 10.1007/s40265-021-01597-9
52. Giossi R, Pani A, Schroeder J, Scaglione F. Exploring the risk of infection events in patients with asthma receiving anti-IL-5 monoclonal antibodies: A rapid systematic review and a meta-analysis. Heliyon 2024; 10 (1): e23725. doi: 10.1016/j.heliyon.2023.e23725
53. Li W, Tang S-C, Jin L. Adverse events of anti-IL-5 drugs in patients with eosinophilic asthma: a meta-analysis of randomized controlled trials and real-world evidence-based assessments. BMC Pulm Med 2024; 24 (1): 70. doi: 10.1186/s12890-024-02885-2
54. Committee for Propietary Medical Products EPAR. Fasenra ® ficha tecnica del medicamento. The European Agency for the evaluation of medicinal products (EMEA). https://ec.europa.eu/health/documents/community-register/2018/20180108139598/anx_139598_es.pdf
55. Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 2013; 132 (5): 1086-1096.e5. doi: 10.1016/j.jaci.2013.05.020
56. Bachert C, Han JK, Desrosiers MY, et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J Allergy Clin Immunol 2022; 149 (4): 1309-1317.e12. doi: 10.1016/j.jaci.2021.08.030
57. Nanzer AM, Maynard-Paquette A-C, Alam V, et al. Long-Term Effectiveness of Benralizumab in Eosinophilic Granulomatosis With Polyangiitis. J Allergy Clin Immunol Pract 2024; 12 (3): 724-732. doi: 10.1016/j.jaip.2024.01.006
58. Wedner HJ, Fujisawa T, Guilbert TW, et al. Benralizumab in children with severe eosinophilic asthma: Pharmacokinetics and long‐term safety (TATE study). Pediatr Allergy Immunol 2024; 35 (3). doi: 10.1111/pai.14092
59. Castro M, Wenzel SE, Bleecker ER, et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med 2014; 2 (11): 879-890. doi: 10.1016/S2213-2600(14)70201-2
60. Markham A. Benralizumab: First Global Approval. Drugs 2018; 78 (4): 505-511. doi: 10.1007/s40265-018-0876-8
61. Naftel J, Eames C, Kerley S, et al. Benralizumab treatment of severe asthma in pregnancy: A case series. J Allergy Clin Immunol Pract 2023; 11 (9): 2919-2921. doi: 10.1016/j.jaip.2023.06.061
62. Zhang J, Kuvelkar R, Murgolo NJ, et al. Mapping and characterization of the epitope(s) of Sch 55700, a humanized mAb, that inhibits human IL-5. Int Immunol 1999; 11 (12): 1935-1944. doi: 10.1093/intimm/11.12.1935
63. Ficha Técnica Reslizumab. https://cima.aemps.es/cima/pdfs/es/ft/1161125001/FichaTecnica_1161125001.html.pdf
64. About severe eosinophilic asthma. CINQAIR.
65. Teva Pharmaceuticals. Reslizumab (Cinqaero): summary of product characteristics. 2016. https://www.ema.europa.eu
66. Respiratory T. Cinqair (reslizumab) injection, for intravenous use: US prescribing information.
67. Deeks ED, Brusselle G. Reslizumab in Eosinophilic Asthma: A Review. Drugs 2017; 77 (7): 777-784. doi: 10.1007/s40265-017-0740-2
68. Murphy K, Jacobs J, Bjermer L, et al. Long-term Safety and Efficacy of Reslizumab in Patients with Eosinophilic Asthma. J Allergy Clin Immunol Pract 2017; 5 (6): 1572-1581.e3. doi: 10.1016/j.jaip.2017.08.024
69. EMA. European public assessment report (EPAR): Cinqaero (reslizumab). 2016. https://www.ema.europa.eu
70. Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/0761033s010lbl.pdf
71. Pfaller B, José Yepes‐Nuñez J, Agache I, et al. Biologicals in atopic disease in pregnancy: An EAACI position paper. Allergy 2021; 76 (1): 71-89. doi: 10.1111/all.14282
72. Bavbek S, Pagani M, Alvarez‐Cuesta E, et al. Hypersensitivity reactions to biologicals: An EAACI position paper. Allergy 2022; 77 (1): 39-54. doi: 10.1111/all.14984
73. Kuruvilla ME, Vanijcharoenkarn K, Shih JA, Lee FE-H. Epidemiology and risk factors for asthma. Respir Med 2019; 149: 16-22. doi: 10.1016/j.rmed.2019.01.014
74. Pepper AN, Hanania NA, Humbert M, Casale TB. How to Assess Effectiveness of Biologics for Asthma and What Steps to Take When There Is Not Benefit. J Allergy Clin Immunol Pract 2021; 9 (3): 1081-1088. doi: 10.1016/j.jaip.2020.10.048
75. Li L, Wang Z, Cui L, Xu Y, Guan K, Zhao B. Anaphylactic risk related to omalizumab, benralizumab, reslizumab, mepolizumab, and dupilumab. Clin Transl Allergy 2021; 11 (4). doi: 10.1002/clt2.12038
76. Pichler WJ. Adverse side‐effects to biological agents. Allergy 2006; 61 (8): 912-920. doi: 10.1111/j.1398-9995.2006.01058.x
77. Hausmann OV, Seitz M, Villiger PM, Pichler WJ. The Complex Clinical Picture of Side Effects to Biologicals. Med Clin North Am 2010; 94 (4): 791-804. doi: 10.1016/j.mcna.2010.03.001
78. Chow TG, Franzblau LE, Khan DA. Adverse Reactions to Biologic Medications Used in Allergy and Immunology Diseases. Curr Allergy Asthma Rep 2022; 22 (12): 195-207. doi: 10.1007/s11882-022-01048-9
79. Larkin HD. Therapy Approved for All Types of Severe Asthma. JAMA 2022; 327 (9): 805. doi: 10.1001/jama.2022.1875
80. Menzies-Gow A, Ponnarambil S, Downie J, Bowen K, et al. DESTINATION: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the long-term safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res 2020; 21 (1): 279. doi: 10.1186/s12931-020-01541-7
81. L. Ramos C, Namazy J. Monoclonal Antibodies (Biologics) for Allergic Rhinitis, Asthma, and Atopic Dermatitis During Pregnancy and Lactation. Immunol Allergy Clin North Am 2023; 43 (1): 187-197. doi: 10.1016/j.iac.2022.07.001
82. Corren J, Parnes JR, Wang L, et al. Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med 2017; 377 (10): 936-946. doi: 10.1056/NEJMoa1704064
83. Lin F, Yu B, Deng B, He R. The efficacy and safety of tezepelumab in the treatment of uncontrolled asthma: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2023; 102 (32): e34746. doi: 10.1097/MD.0000000000034746
84. Menzies-Gow A, Wechsler ME, Brightling CE, et al. Long-term safety and efficacy of tezepelumab in people with severe, uncontrolled asthma (DESTINATION): a randomised, placebo-controlled extension study. Lancet Respir Med 2023; 11 (5): 425-438. doi: 10.1016/S2213-2600(22)00492-1
85. Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2021; 9 (11): 1299-1312. doi: 10.1016/S2213-2600(21)00226-5
86. Iribarren C, Rahmaoui A, Long AA, et al. Cardiovascular and cerebrovascular events among patients receiving omalizumab: Results from EXCELS, a prospective cohort study in moderate to severe asthma. J Allergy Clin Immunol 2017; 139 (5): 1489-1495.e5. doi: 10.1016/j.jaci.2016.07.038
87. Panettieri RA, Sjöbring U, Péterffy A, et al. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir Med 2018; 6 (7): 511-525. doi: 10.1016/S2213-2600(18)30184-X
88. Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N Engl J Med 2021; 384 (19): 1800-1809. doi: 10.1056/NEJMoa2034975

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright (c) 2025 Revista Alergia México