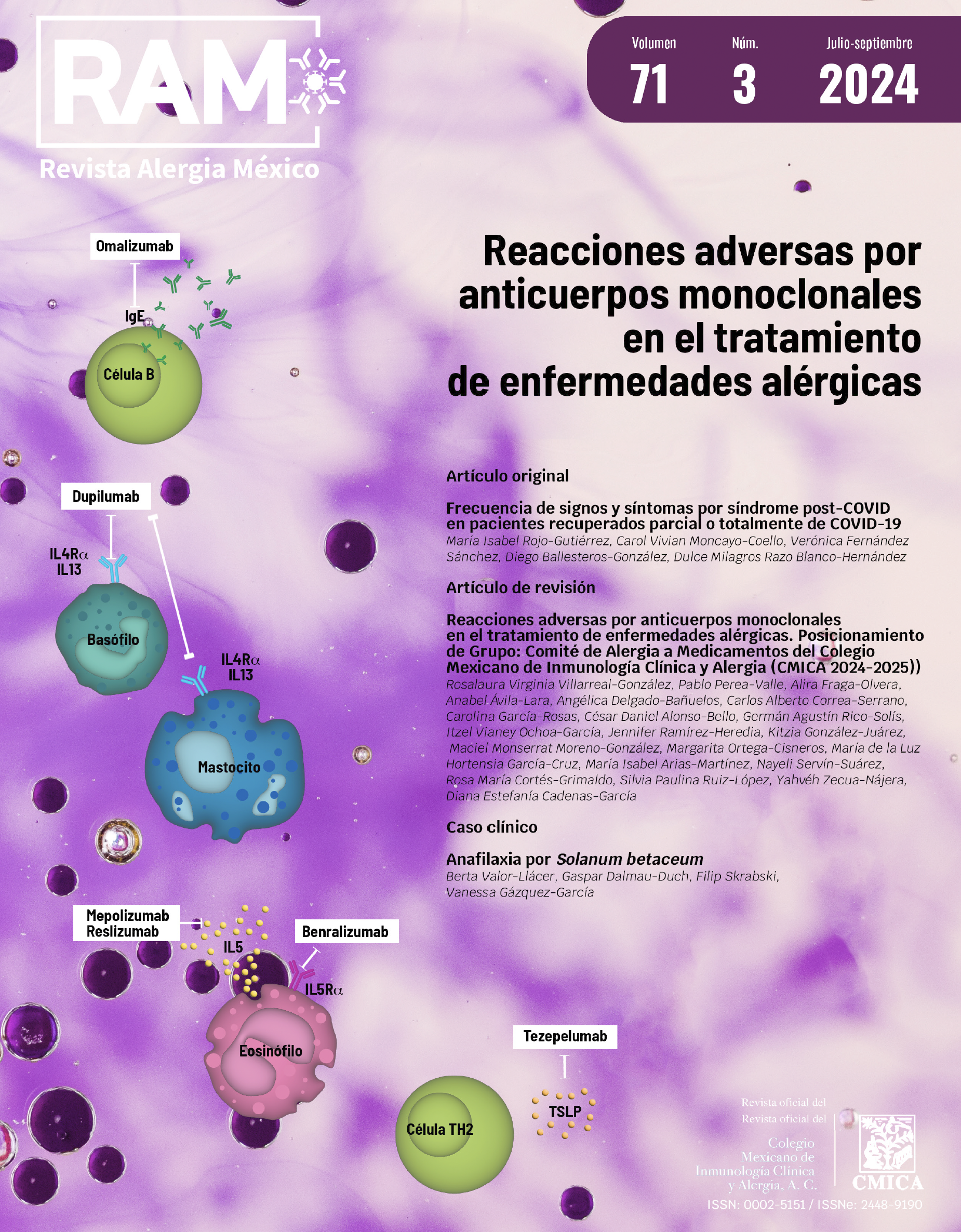
Resumen
Antecedentes: Los anticuerpos monoclonales representan una opción de tratamiento en pacientes con enfermedades alérgicas, autoinmunes, oncológicas, entre otras. Su función consiste en inhibir las interacciones entre moléculas efectoras y sus receptores específicos. Sin embargo, el aumento de su prescripción ha provocado un incremento de las reacciones adversas a medicamentos. Este tipo de reacciones son respuestas dañinas e inesperadas a tratamientos terapéuticos. Se dividen en tipo A, relacionadas con la acción del medicamento y sus interacciones, y tipo B, asociadas con reacciones de hipersensibilidad.
Objetivos: Se llevó a cabo una revisión bibliográfica de la prescripción de anticuerpos monoclonales en el tratamiento de enfermedades alérgicas, abordando la farmacocinética, farmacodinámica, posología, contraindicaciones y reacciones adversas.
Metodología: La búsqueda de información se realizó en las principales bases de datos médicas, acerca de los anticuerpos monoclonales para el tratamiento de enfermedades alérgicas. La búsqueda se limitó a artículos originales, en idioma inglés y español, publicados entre 2014 y 2024. Se describe la actualización de la información relacionada con anticuerpos monoclonales para enfermedades alérgicas, incluyendo su mecanismo de acción, nombre comercial, indicaciones, posología, contraindicaciones y reacciones adversas.
Conclusión: La recopilación de datos acerca de medicamentos biológicos es decisiva para la comprensión integral y actualizada de su prescripción clínica. Conocer las reacciones adversas mejora el entendimiento del diagnóstico y la calidad de la atención médica.
Palabras clave: Anticuerpos monoclonales; Reacción adversa a medicamentos; Reacciones de hipersensibilidad; Farmacocinética; Farmacodinámica; Posología; Contraindicaciones; Reacciones adversas.
Referencias
WHO Expert Committee on Biological Standardization. World Health Organ Tech Rep Ser 2014; (987): 1-266.
2. Mori F, Saretta F, Bianchi A, et al. Hypersensitivity Reactions to Monoclonal Antibodies in Children. Medicina (Buenos Aires) 2020; 56 (5): 232. doi: 10.3390/medicina56050232
3. Ozdemir C. Monoclonal Antibodies in Allergy; Updated Applications and Promising Trials. Recent Pat Inflamm Allergy Drug Discov 2015; 9 (1): 54-65. doi: 10.2174/1872213X09666150223115303
4. Zazzara MB, Palmer K, Vetrano DL, Carfì A, Onder G. Adverse drug reactions in older adults: a narrative review of the literature. Eur Geriatr Med 2021; 12 (3): 463-473. doi: 10.1007/s41999-021-00481-9
5. Pichler WJ. Immune pathomechanism and classification of drug hypersensitivity. Allergy Eur J Allergy Clin Immunol 2019; 74 (8): 1457-1471. doi: 10.1111/all.13765
6. Corominas M, Gastaminza G, Lobera T. Hypersensitivity reactions to biological drugs. J Investig Allergol Clin Immunol 2014; 24 (4): 212-225.
7. Isabwe GAC, Garcia Neuer M, de las Vecillas Sanchez L, Lynch D-M, et al. Hypersensitivity reactions to therapeutic monoclonal antibodies: Phenotypes and endotypes. J Allergy Clin Immunol 2018; 142 (1): 159-170.e2. doi: 10.1016/j.jaci.2018.02.018
8. Food and Drug Administration. XOLAIR® (omalizumab) injection for subcutaneous use initial U.S. Approval 2003. XOLAIR® (omalizumab) injection for subcutaneous use initial U.S. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/103976s5225lbl.pdf
9. Purizaca-Bazán J, Ortega-Martell JA. Bloqueo de inmunoglobulina E en el asma grave. Rev Alerg México 2020; 67. doi: 10.29262/ram.v67i7.777
10. Kavati A, Zhdanava M, Ortiz B, et al. Retrospective Study on the Association of Biomarkers With Real-world Outcomes of Omalizumab-treated Patients With Allergic Asthma. Clin Ther 2019; 41 (10): 1956-1971. doi: 10.1016/j.clinthera.2019.07.021
11. Casale TB, Luskin AT, Busse W, et al. Omalizumab Effectiveness by Biomarker Status in Patients with Asthma: Evidence From PROSPERO, A Prospective Real-World Study. J Allergy Clin Immunol Pract 2019; 7 (1): 156-164.e1. doi: 10.1016/j.jaip.2018.04.043
12. Canonica GW, Rottoli P, Bucca C, et al. Improvement of patient-reported outcomes in severe allergic asthma by omalizumab treatment: the real life observational PROXIMA study. World Allergy Organ J 2018; 11: 33. doi: 10.1186/s40413-018-0214-3
13. Bhutani M, Yang WH, Hébert J, de Takacsy F, et al. The real world effect of omalizumab add on therapy for patients with moderate to severe allergic asthma: The ASTERIX Observational study. Fehrenbach H, ed. PLoS One 2017; 12 (8): e0183869. doi: 10.1371/journal.pone.0183869
14. Damask C, Chen M, Holweg CTJ, Yoo B, et al. Defining the Efficacy of Omalizumab in Nasal Polyposis: A POLYP 1 and POLYP 2 Subgroup Analysis. Am J Rhinol Allergy 2022; 36 (1): 135-141. doi: 10.1177/19458924211030486
15. Seetasith A, Holden M, Hetherington J, et al. Real-world outcomes in patients with chronic spontaneous urticaria receiving omalizumab: insights from a clinical practice survey. Curr Med Res Opin 2024: 1-12. doi: 10.1080/03007995.2024.2354534
16. Wood RA, Togias A, Sicherer SH, et al. Omalizumab for the Treatment of Multiple Food Allergies. N Engl J Med 2024; 390 (10): 889-899. doi: 10.1056/NEJMoa2312382
17. Chebani R, Lombart F, Chaby G, et al. Omalizumab in the treatment of bullous pemphigoid resistant to first-line therapy: a French national multicentre retrospective study of 100 patients. Br J Dermatol 2024; 190 (2): 258-265. doi: 10.1093/bjd/ljad369
18. Ferri N, Bellosta S, Baldessin L, Boccia D, et al. Pharmacokinetics interactions of monoclonal antibodies. Pharmacol Res 2016; 111: 592-599. doi: 10.1016/j.phrs.2016.07.015
19. C K. Omalizumab. 2023. Checar Referencia completa
20. Dantzer JA, Wood RA. Update on omalizumab in allergen immunotherapy. Curr Opin Allergy Clin Immunol 2021; 21 (6): 559-568. doi: 10.1097/ACI.0000000000000781
21. Namazy J, Cabana MD, Scheuerle AE, et al. The Xolair Pregnancy Registry (EXPECT): The safety of omalizumab use during pregnancy. J Allergy Clin Immunol 2015; 135 (2): 407-412. doi: 10.1016/j.jaci.2014.08.025
22. Namazy JA, Blais L, Andrews EB, et al. Pregnancy outcomes in the omalizumab pregnancy registry and a disease-matched comparator cohort. J Allergy Clin Immunol 2020; 145 (2): 528-536.e1. doi: 10.1016/j.jaci.2019.05.019
23. Anderson PO. Monoclonal Antibodies During Breastfeeding. Breastfeed Med 2021; 16 (8): 591-593. doi: 10.1089/bfm.2021.0110
24. Saito J, Yakuwa N, Sandaiji N, et al. Omalizumab concentrations in pregnancy and lactation: A case study. J Allergy Clin Immunol Pract 2020; 8 (10): 3603-3604. doi: 10.1016/j.jaip.2020.05.054
25. Cox L, Platts-Mills TAE, Finegold I, Schwartz LB, et al. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J Allergy Clin Immunol 2007; 120 (6): 1373-1377. doi: 10.1016/j.jaci.2007.09.032
26. Harrison RG, MacRae M, Karsh J, Santucci S, et al. Anaphylaxis and serum sickness in patients receiving omalizumab: reviewing the data in light of clinical experience. Ann Allergy, Asthma Immunol 2015; 115 (1): 77-78. doi: 10.1016/j.anai.2015.04.014
27. Bagnasco D, Canevari RF, Del Giacco S, et al. Omalizumab and cancer risk: Current evidence in allergic asthma, chronic urticaria, and chronic rhinosinusitis with nasal polyps. World Allergy Organ J 2022; 15 (12): 100721. doi: 10.1016/j.waojou.2022.100721
28. Harb H, Chatila TA. Mechanisms of Dupilumab. Clin Exp Allergy 2020; 50 (1): 5-14. doi: 10.1111/cea.13491
29. Matsunaga K, Katoh N, Fujieda S, Izuhara K, et al. Dupilumab: Basic aspects and applications to allergic diseases. Allergol Int 2020; 69 (2): 187-196. doi: 10.1016/j.alit.2020.01.002
30. Food and Drug Administration. DUPIXENT ® (dupilumab) inyection for subcutaneous use Initial. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761055s042lbl.pdf
31. Sitek AN, Li JT, Pongdee T. Risks and safety of biologics: A practical guide for allergists. World Allergy Organ J 2023; 16 (1): 100737. doi: 10.1016/j.waojou.2022.100737
32. Gade A. Dupilumab. StatPearls. 2024. https://www.ncbi.nlm.nih.gov/books/NBK585114/
33. Khamisy-Farah R, Damiani G, Kong JD, Wu J-H, et al. Safety profile of Dupilumab during pregnancy: a data mining and disproportionality analysis of over 37,000 reports from the WHO individual case safety reporting database (VigiBaseTM). Eur Rev Med Pharmacol Sci 2021; 25 (17): 5448-5451. doi: 10.26355/eurrev_202109_26652
34. Bosma AL, Gerbens LAA, Middelkamp‐Hup MA, Spuls PI. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol 2021; 46 (6): 1089-1092. doi: 10.1111/ced.14725
35. Adam DN, Gooderham MJ, Beecker JR, et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J Eur Acad Dermatology Venereol 2023; 37 (6): 1135-1148. doi: 10.1111/jdv.18922
36. Wechsler ME, Ford LB, Maspero JF, et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med 2022; 10 (1): 11-25. doi: 10.1016/S2213-2600(21)00322-2
37. Albader SS, Alharbi AA, Alenezi RF, Alsaif FM. Dupilumab side effect in a patient with atopic dermatitis: a case report study. Biol Targets Ther 2019; 13: 79-82. doi: 10.2147/BTT.S195512
38. Partida-Gaytán A, Torre-Bouscoulet L, Macías MP, Raimondi A, et al. Mepolizumab para el tratamiento de asma grave eosinofílica. Rev Alerg Méx 2021; 67. doi: 10.29262/ram.v67i7.780
39. Food and Drug Administration. NUCALA (Mepolizumab): Highlights of prescribing information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125526Orig1s021,761 122Orig1s011Corrected_lbl.pdf
40. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380 (9842): 651-659. doi: 10.1016/S0140-6736(12)60988-X
41. Moore WC, Kornmann O, Humbert M, et al. Stopping versus continuing long-term mepolizumab treatment in severe eosinophilic asthma (COMET study). Eur Respir J 2022; 59 (1): 2100396. doi: 10.1183/13993003.00396-2021
42. Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021; 9 (10): 1141-1153. doi: 10.1016/S2213-2600(21)00097-7
43. Steinfeld J, Bradford ES, Brown J, et al. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol 2019; 143 (6): 2170-2177. doi: 10.1016/j.jaci.2018.11.041
44. Roufosse F, Kahn J-E, Rothenberg ME, et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: A phase III, randomized, placebo-controlled trial. J Allergy Clin Immunol 2020; 146 (6): 1397-1405. doi: 10.1016/j.jaci.2020.08.037
45. Ullmann N, Peri F, Florio O, et al. Severe Pediatric Asthma Therapy: Mepolizumab. Front Pediatr 2022; 10. doi: 10.3389/fped.2022.920066
46. Liu AY. Infectious Implications of Interleukin-1, Interleukin-6, and T Helper Type 2 Inhibition. Infect Dis Clin North Am 2020; 34 (2): 211-234. doi: 10.1016/j.idc.2020.02.003
47. Dighriri IM, Alnughaythir AI, Albesisi AA, et al. Efficacy and Safety of Mepolizumab in the Management of Severe Eosinophilic Asthma: A Systematic Review. Cureus 2023. doi: 10.7759/cureus.49781
48. Middleton PG, Gade EJ, Aguilera C, et al. ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases. Eur Respir J 2020; 55 (2): 1901208. doi: 10.1183/13993003.01208-2019
49. Vittorakis SK, Giannakopoulou G, Samitas K, Zervas E. Successful and safe treatment of severe steroid depended eosinophilic asthma with mepolizumab in a woman during pregnancy. Respir Med Case Reports 2023; 41: 101785. doi: 10.1016/j.rmcr.2022.101785
50. Ozden G, Pınar Deniz P. May mepolizumab used in asthma correct subfertility? Ann Med. 2021; 53 (1): 456-458. doi: 10.1080/07853890.2021.1900591
51. Domingo Ribas C, Carrillo Díaz T, Blanco Aparicio M, et al. Real World Effectiveness and Safety of Mepolizumab in a Multicentric Spanish Cohort of Asthma Patients Stratified by Eosinophils: The REDES Study. Drugs 2021; 81 (15): 1763-1774. doi: 10.1007/s40265-021-01597-9
52. Giossi R, Pani A, Schroeder J, Scaglione F. Exploring the risk of infection events in patients with asthma receiving anti-IL-5 monoclonal antibodies: A rapid systematic review and a meta-analysis. Heliyon 2024; 10 (1): e23725. doi: 10.1016/j.heliyon.2023.e23725
53. Li W, Tang S-C, Jin L. Adverse events of anti-IL-5 drugs in patients with eosinophilic asthma: a meta-analysis of randomized controlled trials and real-world evidence-based assessments. BMC Pulm Med 2024; 24 (1): 70. doi: 10.1186/s12890-024-02885-2
54. Committee for Propietary Medical Products EPAR. Fasenra ® ficha tecnica del medicamento. The European Agency for the evaluation of medicinal products (EMEA). https://ec.europa.eu/health/documents/community-register/2018/20180108139598/anx_139598_es.pdf
55. Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 2013; 132 (5): 1086-1096.e5. doi: 10.1016/j.jaci.2013.05.020
56. Bachert C, Han JK, Desrosiers MY, et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J Allergy Clin Immunol 2022; 149 (4): 1309-1317.e12. doi: 10.1016/j.jaci.2021.08.030
57. Nanzer AM, Maynard-Paquette A-C, Alam V, et al. Long-Term Effectiveness of Benralizumab in Eosinophilic Granulomatosis With Polyangiitis. J Allergy Clin Immunol Pract 2024; 12 (3): 724-732. doi: 10.1016/j.jaip.2024.01.006
58. Wedner HJ, Fujisawa T, Guilbert TW, et al. Benralizumab in children with severe eosinophilic asthma: Pharmacokinetics and long‐term safety (TATE study). Pediatr Allergy Immunol 2024; 35 (3). doi: 10.1111/pai.14092
59. Castro M, Wenzel SE, Bleecker ER, et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med 2014; 2 (11): 879-890. doi: 10.1016/S2213-2600(14)70201-2
60. Markham A. Benralizumab: First Global Approval. Drugs 2018; 78 (4): 505-511. doi: 10.1007/s40265-018-0876-8
61. Naftel J, Eames C, Kerley S, et al. Benralizumab treatment of severe asthma in pregnancy: A case series. J Allergy Clin Immunol Pract 2023; 11 (9): 2919-2921. doi: 10.1016/j.jaip.2023.06.061
62. Zhang J, Kuvelkar R, Murgolo NJ, et al. Mapping and characterization of the epitope(s) of Sch 55700, a humanized mAb, that inhibits human IL-5. Int Immunol 1999; 11 (12): 1935-1944. doi: 10.1093/intimm/11.12.1935
63. Ficha Técnica Reslizumab. https://cima.aemps.es/cima/pdfs/es/ft/1161125001/FichaTecnica_1161125001.html.pdf
64. About severe eosinophilic asthma. CINQAIR.
65. Teva Pharmaceuticals. Reslizumab (Cinqaero): summary of product characteristics. 2016. https://www.ema.europa.eu
66. Respiratory T. Cinqair (reslizumab) injection, for intravenous use: US prescribing information.
67. Deeks ED, Brusselle G. Reslizumab in Eosinophilic Asthma: A Review. Drugs 2017; 77 (7): 777-784. doi: 10.1007/s40265-017-0740-2
68. Murphy K, Jacobs J, Bjermer L, et al. Long-term Safety and Efficacy of Reslizumab in Patients with Eosinophilic Asthma. J Allergy Clin Immunol Pract 2017; 5 (6): 1572-1581.e3. doi: 10.1016/j.jaip.2017.08.024
69. EMA. European public assessment report (EPAR): Cinqaero (reslizumab). 2016. https://www.ema.europa.eu
70. Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/0761033s010lbl.pdf
71. Pfaller B, José Yepes‐Nuñez J, Agache I, et al. Biologicals in atopic disease in pregnancy: An EAACI position paper. Allergy 2021; 76 (1): 71-89. doi: 10.1111/all.14282
72. Bavbek S, Pagani M, Alvarez‐Cuesta E, et al. Hypersensitivity reactions to biologicals: An EAACI position paper. Allergy 2022; 77 (1): 39-54. doi: 10.1111/all.14984
73. Kuruvilla ME, Vanijcharoenkarn K, Shih JA, Lee FE-H. Epidemiology and risk factors for asthma. Respir Med 2019; 149: 16-22. doi: 10.1016/j.rmed.2019.01.014
74. Pepper AN, Hanania NA, Humbert M, Casale TB. How to Assess Effectiveness of Biologics for Asthma and What Steps to Take When There Is Not Benefit. J Allergy Clin Immunol Pract 2021; 9 (3): 1081-1088. doi: 10.1016/j.jaip.2020.10.048
75. Li L, Wang Z, Cui L, Xu Y, Guan K, Zhao B. Anaphylactic risk related to omalizumab, benralizumab, reslizumab, mepolizumab, and dupilumab. Clin Transl Allergy 2021; 11 (4). doi: 10.1002/clt2.12038
76. Pichler WJ. Adverse side‐effects to biological agents. Allergy 2006; 61 (8): 912-920. doi: 10.1111/j.1398-9995.2006.01058.x
77. Hausmann OV, Seitz M, Villiger PM, Pichler WJ. The Complex Clinical Picture of Side Effects to Biologicals. Med Clin North Am 2010; 94 (4): 791-804. doi: 10.1016/j.mcna.2010.03.001
78. Chow TG, Franzblau LE, Khan DA. Adverse Reactions to Biologic Medications Used in Allergy and Immunology Diseases. Curr Allergy Asthma Rep 2022; 22 (12): 195-207. doi: 10.1007/s11882-022-01048-9
79. Larkin HD. Therapy Approved for All Types of Severe Asthma. JAMA 2022; 327 (9): 805. doi: 10.1001/jama.2022.1875
80. Menzies-Gow A, Ponnarambil S, Downie J, Bowen K, et al. DESTINATION: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the long-term safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res 2020; 21 (1): 279. doi: 10.1186/s12931-020-01541-7
81. L. Ramos C, Namazy J. Monoclonal Antibodies (Biologics) for Allergic Rhinitis, Asthma, and Atopic Dermatitis During Pregnancy and Lactation. Immunol Allergy Clin North Am 2023; 43 (1): 187-197. doi: 10.1016/j.iac.2022.07.001
82. Corren J, Parnes JR, Wang L, et al. Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med 2017; 377 (10): 936-946. doi: 10.1056/NEJMoa1704064
83. Lin F, Yu B, Deng B, He R. The efficacy and safety of tezepelumab in the treatment of uncontrolled asthma: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2023; 102 (32): e34746. doi: 10.1097/MD.0000000000034746
84. Menzies-Gow A, Wechsler ME, Brightling CE, et al. Long-term safety and efficacy of tezepelumab in people with severe, uncontrolled asthma (DESTINATION): a randomised, placebo-controlled extension study. Lancet Respir Med 2023; 11 (5): 425-438. doi: 10.1016/S2213-2600(22)00492-1
85. Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2021; 9 (11): 1299-1312. doi: 10.1016/S2213-2600(21)00226-5
86. Iribarren C, Rahmaoui A, Long AA, et al. Cardiovascular and cerebrovascular events among patients receiving omalizumab: Results from EXCELS, a prospective cohort study in moderate to severe asthma. J Allergy Clin Immunol 2017; 139 (5): 1489-1495.e5. doi: 10.1016/j.jaci.2016.07.038
87. Panettieri RA, Sjöbring U, Péterffy A, et al. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir Med 2018; 6 (7): 511-525. doi: 10.1016/S2213-2600(18)30184-X
88. Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N Engl J Med 2021; 384 (19): 1800-1809. doi: 10.1056/NEJMoa2034975

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Derechos de autor 2025 Revista Alergia México