Abstract
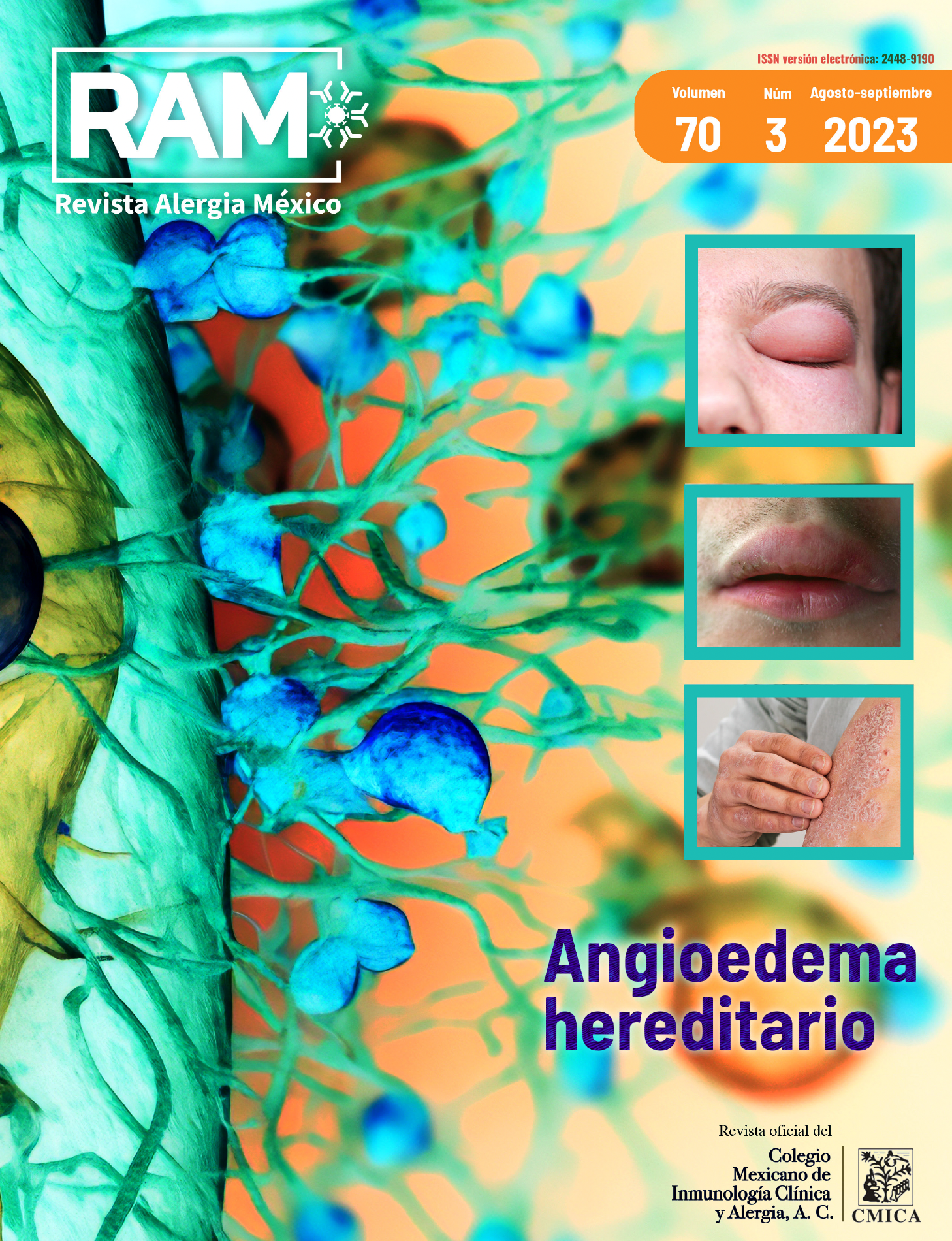
Hereditary angioedema (HAE) is an autosomal dominant disorder, characterized by recurrent, severely limiting angioedema, experiencing poor quality of life. Lanadelumab is a human monoclonal antibody, a specific inhibitor of plasma kallikrein, currently approved for long-term HAE prophylaxis.
Objectives: Report the efficacy of lanadelumab, and the changes in the quality of life in a Mexican patient.
Methodology: A 56-year-old female patient, with HAE type 1, received 300 mg of lanadelumab on day 0, then 300 mg every 2 weeks, quality of life was assessed on day 0, 5, and 10 months after use. with the Angioedema Quality of Life Questionnaire (AE-QoL), Angioedema Control Test (AECT), and with the Angioedema Activity Scoring (AAS).
Results: AECT 0(1 point), 5 months (10 points), 10 months (7 points), AE-Qol 0 (57), 5 months (49), 10 months (46), AAS 0 (32), 5 months (9), 10 months (6).
Discussion: The results confirm a decrease in angioedema activity, as well as a positive impact on the patient's quality of life, with the use of lanadelumab after 10 months of treatment, however it is not yet considered to be controlled based on the EGTC result. It is intended, in the exposed case, to complete a 12-month follow-up.
Conclusion: The purpose of a specific treatment significantly reduces angioedema outbreaks and the integrity of the patient, offering a better quality of life.
References
Diagnostic workup in patients suspected to have HAE. Abbreviations: HAE-1, hereditary angioedema due to C1-inhibitor deficiency; HAE-2, hereditary angioedema due to C1-inhibitor dysfunction; AAE-C1-INH, acquired angioedema due to C1-inhibitor deficiency; HAE nC1-INH, hereditary angioedema with normal C1-inhibitor levels, either due to a mutation in factor XII (FXII) angiopoietin (ANGPTI) or plasminogen (PLG); ACEI-AE, angiotensin-converting enzyme inhibitor-induced angioedema
Zafra H. Hereditary Angioedema: A Review. WMJ. 2022 Apr;121(1):48-53. PMID: 35442579..
The international WAO/EAACI guideline for the management of hereditary angioedema—The 2017 revision and update. M. Maurer et al. .Allergy. 2018;73:1575–1596

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright (c) 2023 Revista Alergia México